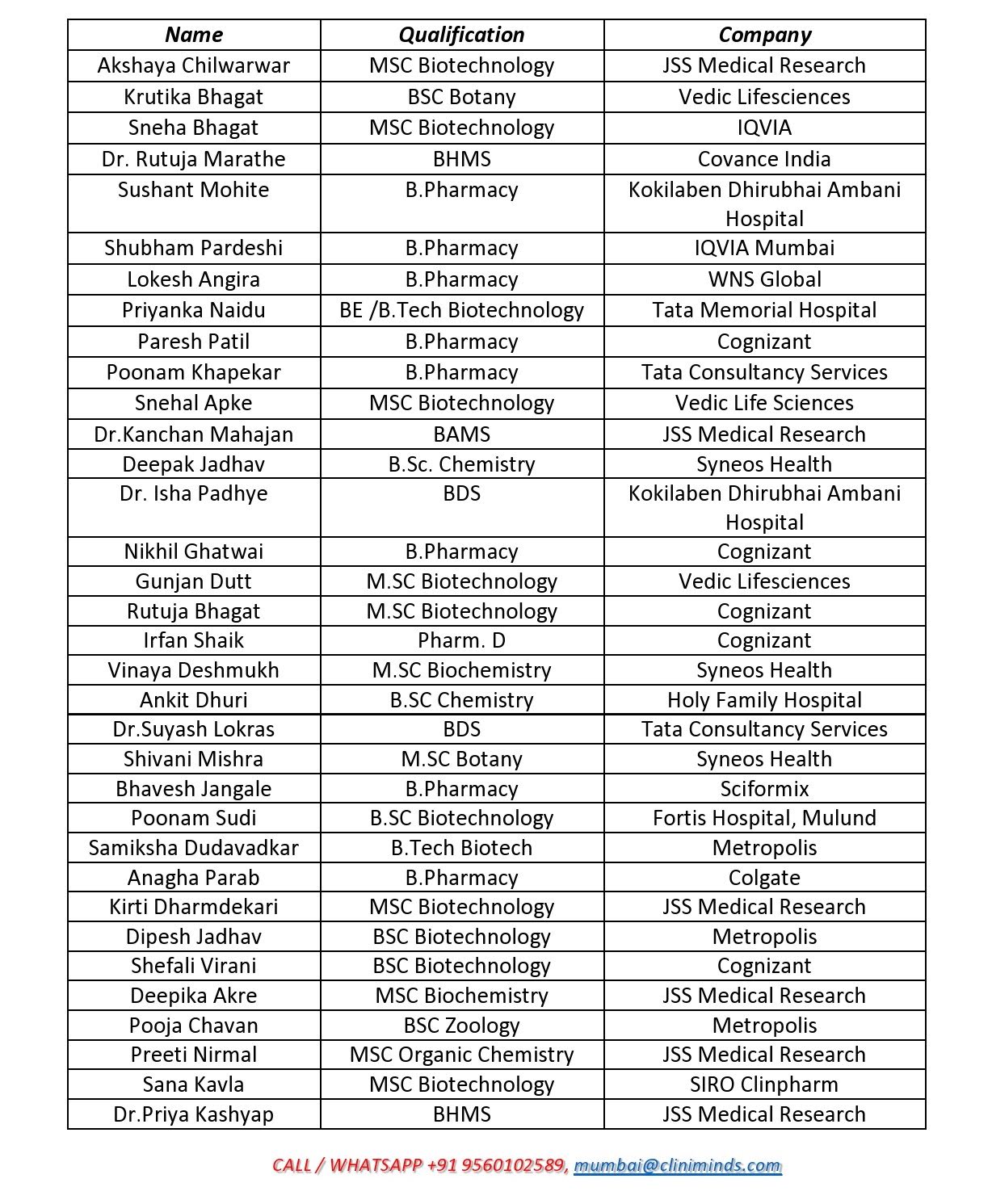
Mumbai Centre
India’s First eSkilling Program in Clinical Research, Pharmacovigilance & Clinical Data Management Certified by Life Sciences Sector Skill Development Council, National Skill Development Corporation, Ministry of Skill Development & Entrepreneurship, Government of India
Cliniminds have entered into the agreement with Life Sciences Sector Skill Development Council (LSSSDC), under the Ministry of Skill Development & Entrepreneurship, Government of India to offer eSkill courses to the eligible candidates. Program offered to you is certified by the LSSSDC. You would be offered Certificate Program in Clinical Research, Pharmacovigilance & Clinical Data Management by LSSSDC in addition to the Post Graduate Diploma offered by Cliniminds.The LSSSDC Certification would increase the value of your qualification, and help in job placements as well.
PROGRAM ACCREDITED BY ACCREDIATION COUNCIL FOR CLINICAL RESEARCH EDUCATION, UNITED STATES OF AMERICA

HIGH QUALITY PROGRAMS WITH EXCELLENT PLACEMENT SUPPORT
FAST TRACK ADVANCED POST GRADUATE DIPLOMA IN CLINICAL RESEARCH & PHARMACOVIGILANCE
FAST TRACK INTENSIVE PROGRAM – 6 WEEKS + 2 WEEKS PROJECT WORK : 100% PLACEMENT PLAN: INTERNSHIPS :
INDUSTRY CERTIFICATION
Following online programs would be offered for the students of akola :
- Post Graduate Diploma in Clinical Research for Medical Devices
- Post Graduate Diploma in Clinical Trials Quality Assurance, GCP Audits & Inspections -
- Post Graduate Diploma in Intellectual Property Rights & Patents -
- Advanced Post Graduate Diploma in Clinical Research -
- Certificate Program in Signal Detection and Risk Mangement
- Diploma in Clinical Research -
- Advanced Post Graduate Diploma in Clinical Research and Clinical Data Management -
- Post Graduate Diploma in Healthcare Insurance Management -
- Post Graduate Diploma in Healthcare Business Management & Administration -
- Post Graduate Diploma in Pharmacovigilance
- Entrepreneurship Program In Clinical Research
- Advanced Post Graduate Diploma in Pharmacovigilance_
- Certificate Program in Conducting & Managing Bioequivalence & Bioavailability Studies
- Post Graduate Diploma in Pharmaceutical Medical Affairs Management
- Certificate Program in Conducting & Managing Clinical Trials for Cancer Patients
- Post Graduate Diploma in Clinical Data Management & Clinical statistical analysis and programming
- Advanced Post Graduate Diploma in Clinical Data Management - (eLearning - Weekend)
- Advanced Post Graduate Diploma in Pharmacovigilance and Medical Writing
- Certificate Program For Clinical Trial Investigators & Site Personnel
- Advanced Post Graduate Diploma in Clinical Data Management - (eLearning - Fulltime)
- ICH GCP Certificate Program in Clinical Research -
- Advanced Post Graduate Diploma in Pharmacovigilance Audits & Inspections
- Advanced Post Graduate Diploma in Pharmacovigilance,Clinical Data Management & Clinical statistical analysis and programming
- Post Graduate Diploma in Health Sciences Business Management
- Advance Post Graduate Diploma in Clinical Research, Clinical Data Management & Clinical statistical analysis and programming
- Certificate Program in Monitoring of Clinical Trials
- Advanced Post Graduate diploma in Pharmacovigilance for Medical Practitioners
- Advanced Post Graduate Diploma in Drug Regulatory Affairs & Pharmacovigilance
- Shree Amar Jain Hospital & Research Institute - Detailed Project Report
- Diploma in Imaging in Clinical Research
- Advance Post Graduate Diploma in Pharmacovigilance and Clinical Data Management
- Medical Review
- Advanced Post Graduate Diploma in Clinical Research, Clinical Data Management & Clinical statistical analysis and programming-Elearning
- Advanced Post Graduate Diploma in Clinical Data Management and Pharmacovigilance
- Advanced Post Graduate Diploma in Clinical Research, Medical and Scientific Content Writing -
- Advanced Post Graduate Diploma in Clinical Research, Biostatistics & Clinical statistical analysis and programming
- Certificate Program in Oracle Clinical.
- Clinical Data Management training on ORACLE CLINICAL SOFTWARE
- Certificate Program in Oracle Argus.
- Certificate Program in Aggregate Reporting -
- Advanced Post Graduate Diploma in Clinical Research, Pharmacovigilance with Oracle Argus Safety Software Training
- Certificate Program in Signal Detection and Risk Management-
- Post Graduate Diploma in Clinical Trials Management
- Advanced Post Graduate Diploma in Clinical Research & Pharmacovigilance...
- Advanced Post Graduate Diploma in Drug Regulatory Affairs & Medical Writing
- Post Graduate Diploma in Bio analytical Techniques
- Advanced Post Graduate Diploma in Pharmacovigilance and Clinical statistical analysis and programming
- Advanced Post Graduate Diploma in Pharmacovigilance and Medical Writing - E-Learning
- Advanced Post Graduate Diploma in Clinical Research & Pharmacovigilance - (eLearning - Weekend)
- Advanced Post Graduate Diploma in Clinical Research & Pharmacovigilance - (eLearning - Fulltime)
- Post Graduate Diploma in Drug Regulatory Affairs, IPR and Patents - E-Learning
- Advanced Post Graduate Diploma in Pharmaceutical Business Analytics - (eLearning - Weekend)
- Advanced Post Graduate Diploma in Biostatistics & Clinical statistical analysis and programming - (eLearning - Weekend)
- Certificate Program in Signal Detection and Risk Management - (eLearning - Weekend)
- Advanced Post Graduate Diploma in Pharmacovigilance - (eLearning - Fulltime)
- Post Graduate Diploma in Clinical Research for Nurses
- Post Graduate Diploma in Clinical Trials Quality Assurance, Auditing & Inspections
- Advanced Post Graduate Diploma in Pharmacovigilance Audits & Inspections
- Advanced Post Graduate Diploma in Clinical Research and Clinical Data Management - (eLearning - Weekend)
- Certificate Program in Good Laboratory Practice -
- Certificate Program in Quality Assurance in Clinical Research
- Advanced Post Graduate Diploma in Pharmacovigilance -
- Certificate Program in Aggregate Reporting - (eLearning - Weekend)
- Certificate Program in Aggregate Reporting
- Certificate Program in Signal Detection and Risk Management
- Advanced Post Graduate Diploma in Clinical Research, Biostatistics & Clinical statistical analysis and programming
- Advanced Post Graduate Diploma in Clinical Research, Clinical Data Management & Clinical statistical analysis and programming - (eLearning - Fulltime)
- Advanced Post Graduate Diploma in Clinical Research, Biostatistics & Clinical statistical analysis and programming - E Learning
- Advanced Post Graduate Diploma in Clinical Data Management, Biostatistics & Clinical statistical analysis and programming
- Certificate Program in Medical and Scientific Content Writing - (eLearning - Weekend)
- Post Graduate Diploma in Drug Regulatory Affairs - Class Room
- Advanced Post Graduate Diploma in Drug Regulatory Affairs - (eLearning - Weekend)
- Certificate Program in Pharmacovigilance Literature Search Screening
- Certificate Program in Pharmacovigilance Literature Search Screening
- Post Graduate Certificate Program In Drug Safety and Medical Review For Physicians
- Post Graduate Certificate Program In Drug Safety and Medical Review For Physicians -
- Post Graduate Certificate Program In Drug Safety and Medical Review For Physicians
- Post Graduate Diploma in Drug Regulatory Affairs -
- 21 CFR PART 11
- GxP (GCP, GMP, GLP, GDocP)
- ICH - GCP Certification & New Drugs & Clinical Trial Rules 2019
- Post Graduate Certificate Program In Drug Safety and Medical Review For Physicians - (eLearning - Weekend)
- Advanced Post Graduate Diploma in Clinical Research Pharmacovigilance & Clinical Data Management - (eLearning - Fulltime)
- Certificate Program in Conducting & Managing Clinical Trials for Cancer Patients-E-Learning
- Advanced Post Graduate Diploma in Clinical Research - (eLearning - Fulltime)
- Advanced Post Graduate Diploma In Biostatistics,Clinical statistical analysis and programming and CDISC/SDTM- Elearning
- Post Graduate Diploma in Clinical Trials & Pharmacovigilance Quality Assurance, Auditing & Inspections- Elearning
- Advanced Post Graduate Diploma in Clinical Research Pharmacovigilance & Clinical Data Management-Distance Learning
- POST GRADUDATE DIPLOMA IN GXP (GLP/GCP/GMP/CSV/21CFR Part 11)-Online
- POST GRADUDATE DIPLOMA IN GXP (GLP/GCP/GMP/CSV/21CFR Part 11)-Elearning
- Advanced Post Graduate Diploma in Clinical Research Pharmacovigilance & Clinical Data Management - (eLearning - Weekend)
- Advanced Post Graduate Diploma in Clinical Research and Clinical Data Management - (eLearning - Fulltime)
- Advanced Post Graduate Diploma in Drug Regulatory Affairs & Pharmacovigilance - (eLearning - Fulltime)
- Post Graduate Diploma in Clinical Trials Management-Elearning
- Advanced Post Graduate Diploma in Clinical Research & Drug Regulatory Affairs - (elearning - Weekend)
- Biostatistics Clinical statistical analysis and programming CDISC SDTM - (elearning - Weekend)
- Biostatistics Clinical statistical analysis and programming CDISC SDTM - (elearning)
- Clinical Research, Biostatistics Clinical statistical analysis and programming CDISC/SDTM - (elearning - Weekend)
- Clinical Research, Biostatistics Clinical statistical analysis and programming CDISC/SDTM - (elearning)
- Advanced Post Graduate Diploma in Clinical Research - (eLearning - Weekend)
- Advanced Post Graduate Diploma in Pharmacovigilance - (eLearning - Weekend)
- Advanced Post Graduate Diploma in Clinical Research Pharmacovigilance & Clinical Data Management -D
- Advanced Post Graduate Diploma in Clinical Research & Pharmacovigilance
- Post Graduate Certificate Program In Drug Safety and Medical Review For Physicians
- Advanced Post Graduate Diploma in Pharmacovigilance
- Advance Post Graduate Diploma in Clinical Research, Clinical Data Management & Clinical statistical analysis and programming
- Certificate Program in Aggregate Reporting
- Certificate Program in Signal Detection and Risk Management
- Post Graduate Diploma in Medical and Scientific Content Writing
- Advanced Post Graduate Diploma in Biostatistics & Clinical statistical analysis and programming
- POST GRADUDATE DIPLOMA IN GXP (GLP/GCP/GMP/CSV/21CFR Part 11)
- Advance Post Graduate Diploma in Clinical Research, Clinical Data Management & Clinical statistical analysis and programming
- Certificate Program in Signal Detection and Risk Management
- Advanced Post Graduate Diploma in Pharmaceutical Business Analytics
- Post Graduate Certificate Program In Drug Safety and Medical Review For Physicians
- Advanced Post Graduate Diploma in Biostatistics & Clinical statistical analysis and programming
- POST GRADUDATE DIPLOMA IN GXP (GLP/GCP/GMP/CSV/21CFR Part 11)
- Advanced Post Graduate Diploma in Clinical Research, Medical and Scientific Content Writing - (eLearning - Weekend)
- Advanced Post Graduate Diploma in Pharmacovigilance & Medical and Scientific Content Writing - (eLearning - Weekend)
- Advanced Post Graduate Diploma in Clinical Research & Drug Regulatory Affairs-
- ICH-GCP PRINCIPLES AND NEW DRUGS & CLINICAL TRIAL RULES, 2019 - Bangalore
- Advanced Post Graduate Diploma in Clinical Research, Clinical Data Management & Clinical statistical analysis and programming - (eLearning - Weekend)
- Certificate Program in Clinical Research & Pharmacovigilance
- Certificate Program in Clinical Research & Clinical Data Management
- Certificate Program in Clinical Research, Pharmacovigilance & Clinical Data Management
- Certificate Program in Pharmaceutical Business Analytics - (eLearning - Weekend)
- Certificate Program in Clinical Trials Monitoring - (eLearning - Weekend)
- Advanced Gynaecological Ultrasound
- Essentials of Early Pregnancy Ultrasound International
- Fetal Cardiac Ultrasound
- Fundamentals Gynaecological Ultrasound International
- Fundamentals Obstetric Ultrasound International
- Fundamentals of Vascular Ultrasound International
- Imaging Science and Technology International
- Advanced Gynaecological Ultrasound
- Advanced Obstetric Ultrasound
- Advanced Vascular Ultrasound International
- Imaging Science and Technology International
- Fundamentals of Andrology for Clinicians International
- Fundamentals of Human Reproduction International
- Essentials of Early Pregnancy Ultrasound International
- Gynaecological Ultrasound for Advanced Practitioners International
- Fundamentals of Gynaecological Ultrasound International
- Certificate Program in Drug Regulatory Affairs, Intellectual Property Rights and Patents -
- Certificate Program in Clinical Research
- EU MDR Training Program
- MEDICAL AND EDUCATION ACADMEY, U.K.
- ICH - GCP Certification & New Drugs & Clinical Trial Rules 2019 - 2 March 2021
- Certificate Program in Healthcare Decision Analytics - (eLearning - Weekend)
- Advanced Post Graduate Diploma in Pharmacovigilance - Ultragenic
- Certificate Program in Conducting & Managing Bioequivalence & Bioavailability Studies - (eLearning - Weekend)
- Certificate Diploma in Pharmacovigilance - Saveo
- Advanced Post Graduate Diploma in Clinical Research and Clinical Data Management
- PV Training and SOP Discussion - Pharma Pharmaceutical Industries (PPI)
- Clinical Research Training - Internal Employee
- Advanced Post Graduate Certificate Program in Drug Safety Management - (eLearning - Weekend)
- ICH - GCP Certification & New Drugs & Clinical Trial Rules 2019 - Paramitha Hospitals
- Advanced Post Graduate Diploma in Drug Regulatory Affairs & Pharmacovigilance - (eLearning - Weekend)
- ADVANCED POST GRADUATE DIPLOMA IN DRUG REGULATORY AFFAIRS & PHARMACOVIGILANCE - 01
- ADVANCED POST GRADUATE DIPLOMA IN CLINICAL RESEARCH, CLINICAL DATA MANAGEMENT & Clinical statistical analysis and programming - 01
- GCP Training - 4 June 2022
- ICH GCP Certificate Program In Clinical Research For Medical Practitioners -
- Post Graduate Diploma in Medical and Scientific Content Writing -
- Post Graduate Diploma in cGMP – Good Manufacturing Practices
- Certificate Program in Clinical Trial Regulations & GCP in Europe & UK
- Certificate Program in Clinical Trials Auditing & Inspection
- Advanced Post Graduate Diploma in Clinical Research & Pharmacovigilance -
- Advanced Post Graduate Diploma in Clinical Research & Drug Regulatory Affairs -
- Advanced Post Graduate Diploma in Biostatistics & Clinical statistical analysis and programming
- Advanced Post Graduate Diploma in Clinical Data Management, Biostatistics & Clinical statistical analysis and programming
- Advanced Post Graduate Diploma in Clinical Data Management-Online
- Advanced Post Graduate Diploma in Clinical Research & Bioequivalence & Bioavailability Studies
- Advanced Post Graduate Diploma in Drug Regulatory Affairs ,Intellectual Property Rights and Patents
- Advanced Post Graduate Diploma in Drug Regulatory Affairs & Pharmacovigilance
- Cliniminds would also provide the wide range of Corporate Training Solutions for the professionals working in the pharmaceutical companies, CROs and hospitals. Cliniminds offers corporate clinical research training solutions and workshops in the following areas :
- ICH-GCP Clinical Research
- Conduct & Management of Clinical Trials
- Monitoring
- Pharmacovigilance
- Regulatory Affairs
- Quality Assurance in Clinical Research
- Auditing & Inspections of Clinical Trials
- Medical & Scientific Writing
For more information, please contact:
Cliniminds Mumbai
+91-9560102589, +91-9599977492, +91-9310485979, +91-9810068241C/O Awfis Vashi, 18th Floor, Cyber One, Plot No.4 & 6, Sector 30A, Vashi, MUMBAI, India, 400703
Email us your queries : mumbai@cliniminds.com



