Life Sciences Sector Skill
Development Council Program
Fulltime - 4 Months Live eLearning
Batch Commencement - Monday, 16th March 2026
Registration Closing Soon
11,500+ Cliniminds Alumni
Eligibility - B.Pharmacy, M.Pharmacy, Doctor of Pharmacy, BSC, MSC, BDS, BHMS, BAMS, B.Tech / M.Tech Biotech, Biomedical, Life Sciences.
Life Sciences Sector Skill
Development Council Program
Fulltime - 4 Months Live eLearning
Batch Commencement - Monday, 16th March 2026
Registration Closing Soon
11,500+ Cliniminds Alumni
Eligibility - B.Pharmacy, M.Pharmacy, Doctor of Pharmacy, BSC, MSC, BDS, BHMS, BAMS, B.Tech / M.Tech Biotech, Biomedical, Life Sciences.
Free Add On Clinical Data Management / Pharmacovigilance with All Live eLearning Programs
Program Inclusions
- Live Interactive eLearning Sessions
- Hands on Software Training - Pharmacovigilance Safety Database, Clinical Data Management, eTMF.
- Assured Job Interviews - Campus Placements.
- Dedicated Placement Cell - 360° Placement Support.
- Pre Placement Training - CV Writing, Mock Interviews, English Communication, Aptitude Test Training
- Access to LMS – 24x7 - Recordings, PPT, Notes, eBooks.
- Best in the Class Placements in Life Sciences Industry
- Since 2004 – Over 8,000+ Placed Students
Certifications
-
1Life Sciences Sector Skill Development Council.
-
2Advanced PG Diploma – Cliniminds - Program Accredited by Accreditation Council for Clinical Research Education, United States of America.
-
3Internship Certification – www.thinki.in
-
4ICH GCP Certificate – Cliniminds
Free Add On Clinical Data Management / Pharmacovigilance with All Live eLearning Programs
- Drug Discovery
- Overview of Clinical Research
- Regulations & Guidelines in Clinical Research
- Roles & Responsibilities of Key Stakeholders
- Preparation & Planning of Clinical Trials
- Essential Documentation in Clinical Research & Regulatory Submission
- Study Start Up Process
- Clinical Monitoring Essentials
- Compliance, Auditing & Quality Control in Clinical Research
- Overview of Clinical Research
- Pharmacology – General Principles
- Introduction to Pharmacovigilance
- Pharmacovigilance Regulations
- Hands on Training on Case Processing and Report Generation
- Safety Reporting and Processing ICSR
- Aggregate Reports, Signal Detection & Risk Management
- Documents in Pharmacovigilance
- Audits & Inspections
- Advanced Pharmacovigilance & Analytics
- Overview of Clinical Data Management
- Clinical Data Integrity
- Data Management Plan
- Design of Case Report Form
- CRF Tracking
- Electronic Data Capture
- Data Entry Guidelines
- Edit Check Creation, Validation, Programming
- Discrepancy Management
- Data Transfer in Clinical Data Management
- Medical Coding Dictionaries
- Laboratory Test in Clinical Trials
- Creating Reports
- Adverse Event Reporting & Reconciliation
- Audit Trail
- Database Lock
- Quality Assurance in Clinical Data Management
- QMS, Audit & Inspection, SOP Compliance
- Hands on Software Training
- Drug Development Process & Introduction to Clinical Research
- Introduction to Regulatory Affairs, Terms & Terminologies & Abbreviations
- Chemistry, Manufacturing & Controls (CMC)
- Drug Master File
- Regulatory, Filing System for DMF in Different Countries
- ICH – Introduction, Objectives & Guidelines
- CTD Introduction - Module 1 to 5
- Regulatory Filing Systems in Europe
- Regulatory Filing Systems in US
- Registration Procedures in Rest of the World
- Validations
- Audits & Compliances
- Regulatory Operations
- Pre Publishing
- Publishing
- Final Submission via Gateways
- Practical Approach towards Overall Dossier Preparation & Submission
- Pharmacovigilance Regulations
- Indian Pharmaceutical Regulations
- Introduction to Patent Regime
- Introduction to Patents
- Trademarks
- Copyright
- Industrial designs
- Trade Secrets
- Geographical indications
- Types of Patent
- Patentability criteria
- Patentable subject matter/Non Patentable subject matter
- Anatomy of Patent
- Term of a Patent Expiry calculation of patents.
- Inventor ship
- Duty of Disclosure
- Timelines
- Types of Searches
- Various Search Engines
- Performing a Search (Dummy Strategy) & Advantages of patent search
- International Patent classification Patent
- Introduction to patent drafting
- Specification and Description
- Written description, Enablement and Best mode requirement
- Claims
- Clinical statistical analysis and programming Introduction
- Infile option
- Methods to read data
- Import Export of data
- Set and where statements
- Libraries
- If then Else statement
- If then and Loop
- Addition of data
- Merging of data
- Functions in Clinical statistical analysis and programming
- Procedures in Clinical statistical analysis and programming
- SQL Overview
- Macro Overview
- ODS(Output Delivery System)
- Introduction to CDISC
- Overview of CT Protocol, SAP, CRF, TFL shell
- Sample CRF Annotation activity
- Overview of SDTM Implementation guide
- Overview of ADaM Implementation guide
- Preparation of Dataset specification (SDTM + ADaM)
- Sample SDTM creation
- Sample ADaM creation
- Creation of .XPT files for Regulatory submission
- Sample TFLs creation
- Overview of P21 tool for validation of data
- Overview of University free Clinical statistical analysis and programming version usage to perform above
- activities on dummy project
- Overview of define.xml and final regulatory submission package


Free Add On Clinical Data Management / Pharmacovigilance with All Live eLearning Programs
Cliniminds Delhi NCR - Cliniminds Delhi NCR - Noida B 602, KLJ Noida One, 6th Floor, Sector 62, Noida, Uttar Pradesh, India 201309
Cliniminds Mumbai - Awfis Vashi, 18th Floor, Cyber One, Sector 30A, Vashi, Mumbai, India, 400703
Cliniminds Bangalore - BHIVE Workspace Platinum Indiranagar, 271, 6th Main Rd, HAL 2nd Stage, Motappapalya, Indiranagar, Bengaluru, Karnataka 560038
Cliniminds Kerala 1 - MWT Global Academy Pvt. Ltd, 37/1989 A, Bhattathiripad Road, Opp. Elamkulam Kavalakkal Temple, Off. Kaloor – Kadavanthra Road, Kaloor, Kochi – 682 017, Kerala, India Tel: +91 9072369008, 8129100876
Cliniminds Kerala 2 - MWT Global Academy Pvt. Ltd, Third Floor, Olive Residency Building, Opp. Caritas Hospital, Thellakom, Kottayam, Kerala 686630 Tel: +91 8129823785, 8129100876
Cliniminds West Bengal - Netaji Subhas Chandra Bose Cancer Hospital, 3081 Nayabad, Near 1B Bus Stand, New Garia, Kolkata, West Bengal, India, 700094
Free Add On Clinical Data Management / Pharmacovigilance with All Live eLearning Programs
Navigating The Maze: A Beginner’s Guide To Drug Development And Clinical Research
The journey of a new drug, from its initial discovery to reaching patients, is a long and complex one. Drug development and clinical research are the cornerstones of this process, ensuring the safety and efficacy of new treatments before they become widely available. This blog will serve as your roadmap, explaining the essential aspects of this intricate world.
Unveiling the Process: Drug Development and Clinical Trials
Drug development starts with identifying a target molecule and progresses through various stages:
- Preclinical research: Conducted in laboratories and using animal models to assess the drug's basic properties and safety.
-
Clinical trials: Involve testing the drug on human volunteers in a series of meticulously designed phases:
- Phase I: Evaluates safety and dosage in a small group of healthy volunteers.
- Phase II: Assesses the drug's effectiveness in patients with the targeted condition and refines the dosage regimen.
- Phase III: Confirms the drug's efficacy and safety in a larger patient population compared to existing treatments or placebo.
- Phase IV: Monitors the drug's long-term safety and effectiveness after its approval for marketing.
Navigating the Regulatory Landscape
Clinical trials must adhere to stringent regulations and guidelines set by various authorities worldwide. Some key regulatory bodies include:
- USA: Food and Drug Administration (FDA)
- India: Central Drugs Standard Control Organization (CDSCO)
- Europe: European Medicines Agency (EMA)
- UK: Medicines and Healthcare products Regulatory Agency (MHRA)
- China: National Medical Products Administration (NMPA)
- Japan: Pharmaceuticals and Medical Devices Agency (PMDA)
A Symphony of Stakeholders
Several key players contribute to the success of clinical research:
- Regulators: Define and enforce regulations for conducting clinical trials.
- Sponsors: Finance and oversee the trials, usually pharmaceutical companies or research institutions.
- Ethics committees/IRBs: Review and approve study protocols to ensure ethical conduct and participant safety.
- Investigators: Conduct the trials and collect data at clinical research sites.
The Compass of Ethics: ICH GCP Principles
The International Council for Harmonisation (ICH) Good Clinical Practice (GCP) principles provide a global ethical and scientific standard for designing, conducting, recording, and reporting clinical trials.
Documentation: The Lifeline of Research
Clinical research relies heavily on comprehensive documentation, including:
- Investigator's Brochure (IB): Summarizes preclinical and clinical data about the drug.
- Clinical trial protocol: Outlines the study design, procedures, and data collection methods.
- Informed consent form: Provides participants with detailed information about the study and their rights.
- Case report forms (CRFs): Capture data on each participant throughout the trial.
Vigilance is Key: Drug Safety Reporting
Identifying and reporting adverse events associated with a drug is crucial throughout its development and post-marketing surveillance.
Ethics: The Bedrock of Research
Maintaining the highest ethical standards is paramount in clinical research. Key principles include:
- Informed consent: Participants must make a voluntary and informed decision to participate.
- Minimizing risk: Risks to participants must be minimized and justified by the potential benefits.
- Data privacy: Participant data must be kept confidential.
- Transparency: Research findings should be reported accurately and transparently.
Charting Your Course: Job Roles in Clinical Research
The clinical research landscape offers diverse career opportunities, including:
- Clinical research associate (CRA): Manages and monitors clinical trials at research sites.
- Data manager: Ensures the accuracy and completeness of clinical trial data.
- Medical monitor: Oversees the medical aspects of a clinical trial and ensures participant safety.
- Clinical trial manager: Coordinates all aspects of a clinical trial.
- Biostatistician: Analyzes clinical trial data to draw meaningful conclusions.
The Global Landscape: Market Size and Clinical Trial Activity
The global clinical research market is expected to reach $76.8 billion by 2027, with significant activity in:
- USA: Largest market for clinical trials - The clinical research market size in the USA is estimated to be around USD 23.83 billion to USD 35.1 billion in 2022, with a projected growth reaching USD 39.62 billion by 2032. This represents a Compound Annual Growth Rate (CAGR) of 4.88% to 4.96% during the forecast period.
- Europe & U.K.: The European & U.K. clinical research market was valued at USD 14.8 billion in 2022 and is expected to reach USD 23.5 billion by 2032, with a CAGR of 5.8% during the forecast period.
- India: Emerging market with increasing clinical trial activity - As of 2023, the Indian clinical research market is valued at approximately USD 1.55 to 2.00 billion. Projected growth: Experts anticipate the market to reach USD 8.36 billion by the end of FY2027, indicating a remarkable Compound Annual Growth Rate (CAGR) of 8.64%. This growth signifies the sector's increasing appeal and potential.
- China: Rapidly growing market with a focus on domestic drug development. As of 2023, the Chinese clinical research market is valued at approximately USD 20.8 billion, making it the second-largest market globally after the United States.
- Japan: Stringent regulatory environment but a significant market for innovative drugs. As of 2023, the Japanese clinical trials market is valued at approximately USD 7.8 billion to USD 9.2 billion. This positions it as the fourth-largest market globally, trailing behind the US, China, and Europe.
Multinational Pharma Companies Involved in Clinical Trials
- AbbVie: A research-based biopharmaceutical company with a global reach. They focus on developing and commercializing innovative medicines in areas like immunology, oncology, neuroscience, and virology.
- AstraZeneca: A British-Swedish multinational pharmaceutical and biotechnology company. They are involved in the development, production, and marketing of prescription drugs, vaccines, and over-the-counter medicines.
- Bayer: A German multinational pharmaceutical and chemical company. They are involved in the development, manufacture, and marketing of human and animal pharmaceuticals, crop protection products, and non-prescription drugs.
- Boehringer Ingelheim: A German multinational pharmaceutical company headquartered in Ingelheim am Rhein. They are focused on developing innovative medicines for unmet medical needs.
- Bristol Myers Squibb: An American multinational pharmaceutical company. They develop, manufacture, and sell pharmaceuticals, biologics, and medical devices.
- Eli Lilly and Company: An American pharmaceutical company headquartered in Indianapolis, Indiana. They are involved in the development, manufacture, and marketing of human and animal pharmaceuticals.
- F. Hoffmann-La Roche: A Swiss multinational pharmaceutical company headquartered in Basel. They are involved in the development, manufacture, and marketing of pharmaceuticals, diagnostics, and medical devices.
- GlaxoSmithKline: A British multinational pharmaceutical company headquartered in London, England. They are involved in the research, development, manufacture, and distribution of prescription medicines, vaccines, and over-the-counter healthcare products.
- Johnson & Johnson: An American multinational pharmaceutical and medical devices corporation. They are involved in the research, development, manufacture, and sale of pharmaceuticals, medical devices, consumer healthcare products, and biotechnology products.
- Merck & Co., Inc.: An American multinational pharmaceutical company headquartered in Whitehouse Station, New Jersey. They are involved in the research, development, manufacture, and distribution of prescription medicines, vaccines, and animal health products.
- Novartis: A Swiss multinational pharmaceutical company headquartered in Basel. They are involved in the research, development, manufacture, and marketing of prescription pharmaceuticals, generics, eye care products, and over-the-counter medicines.
- Pfizer: An American multinational pharmaceutical and biotechnology company headquartered in New York City. They are involved in the research, development, manufacture, and distribution of prescription medicines, vaccines, and over-the-counter healthcare products.
- Roche: A Swiss multinational pharmaceutical company headquartered in Basel. They are involved in the research, development, manufacture, and marketing of pharmaceuticals, diagnostics, and medical devices.
- Sanofi: A French multinational pharmaceutical company headquartered in Paris. They are involved in the research, development, manufacture, and distribution of prescription medicines, vaccines, and consumer healthcare products.
Some Important Global CROs
- Accenture: A multinational professional services company headquartered in Dublin, Ireland. They offer a wide range of services, including clinical research services.
- Covance: An American contract research organization headquartered in Princeton, New Jersey. They offer a wide range of clinical research services, including clinical trial management, regulatory affairs, and bioanalytical services.
- ICON plc: An Irish multinational clinical research organization headquartered in Dublin. They offer a wide range of clinical research services, including clinical trial management, functional source monitoring, and data management.
- IQVIA: An American multinational company that provides information and technology solutions to the healthcare industry. They offer a wide range of clinical research services, including clinical trial design, data management, and analytics.
- Labcorp: An American clinical laboratory services company headquartered in Burlington, North Carolina. They offer a wide range of clinical research services, including clinical trial management, biomarker testing, and genomics.
- Parexel: An American multinational contract research organization headquartered in Waltham, Massachusetts. They offer a wide range of clinical research services.
The global clinical trials industry size is over US$50 billion, with a growth rate of over 12% per annum. Clinical research and allied subsectors are rapidly growing industries globally, requiring highly specialized and skilled professional workforce in the areas of Clinical Research, Clinical Data Management, and Pharmacovigilance. Over 10,000 fresher positions are created every year in the clinical trials and allied sectors in India alone. India's share in the clinical trials and related services outsourcing is approximately US$3 billion. The sector employs over 50,000+ highly skilled professionals. Key global markets like China, Southeast Asia, Europe, and America continue to grow in double digits.
India is now clearly positioned as the preferred outsourcing destination for clinical trials, clinical data management, pharmacovigilance, and related services. India is attracting major pharmaceutical, R&D organizations, and CROs globally. India today offers excellent IT infrastructure, skilled manpower, and cost-efficient solutions in this field, backed up by a strong regulatory environment. Most global companies now have a presence in India, and several other companies are in the process of setting up business in India.
Recent Recruiters at Cliniminds for Placements
- Bio Clinica
- Pharmalex
- Clinchoice
- Kokilaben Dhirubhai Ambani Hospital
- PVPI Government of India
- Navitas Lifesciences
- Notorox Research
- Phamax
- Mascot Spincontrol
- Tata Memorial Hospital
- Novotech
- Cliantha Research
- Eversana Life Sciences
- Fortrea
- LabCorp
- Vasta Bio – Informatics
- Sun Pharma
- IQVIA
- Syneos Health
- Wipro
- Sciformix
- Clario
- JSS Medical Research
- TCS
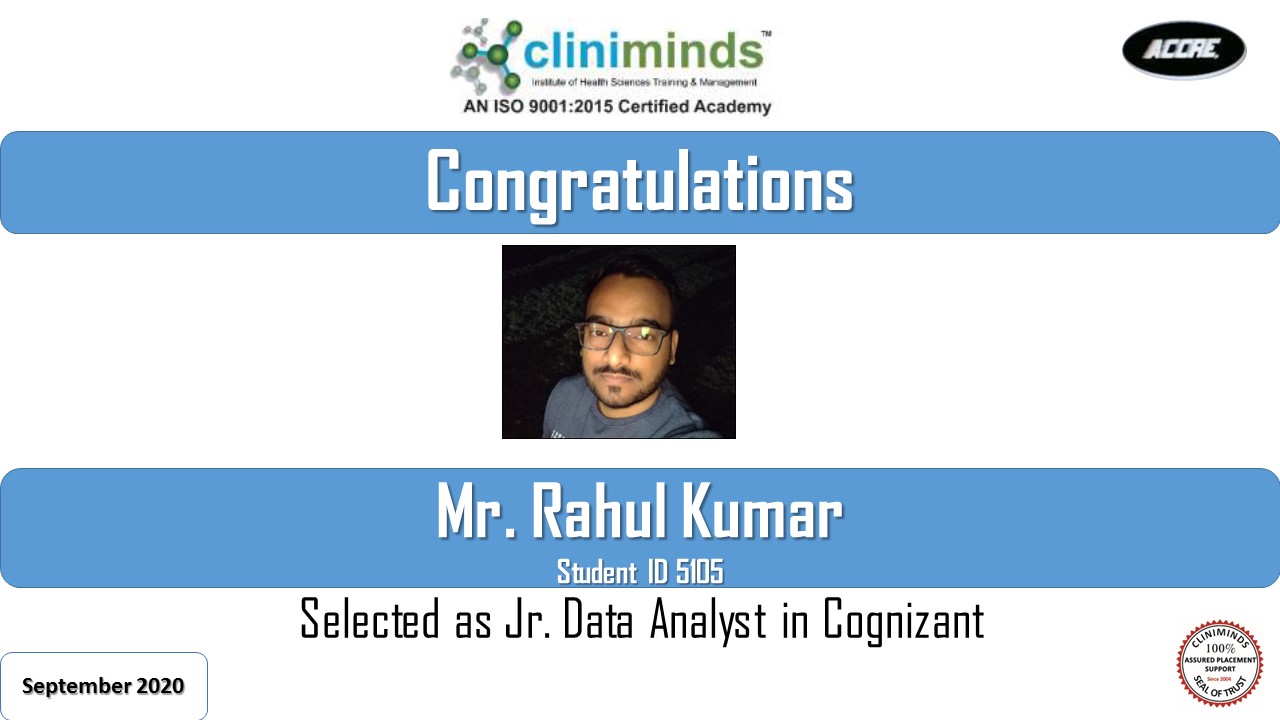
- Cognizant
- Accenture
- Inventurus Knowledge Solution
- Advarra
- APCER
- Merck
- DDReg
- Symphony AI
- Macleods Pharmaceuticals
- Awinsa Lifesciences
- Covance
- Paraxel
- Soterius Lifesciences
- Vedic Lifesciences
- Tech Observer
- Infoclin Research
- Vitality Labs
- Novartis
Entry Level Career Options
- Clinical Research Coordinator
- Clinical Trial Assistant
- Clinical Research Associate
- Pharmacovigilance Associate
- Drug Safety Associate
- Pharmacovigilance Officer
- Clinical Data Associate
- Clinical Data Analyst
- Junior Data Analyst
- Trainee Junior Data Analyst
- Clinical Data Coordinator
- eTMF Specialist
Job Roles And Description
- Independent, proactive work to set up and monitor studies, complete reports and maintain documentation
- Submitting protocol, consent documents for ethics/IRB approval, as well as preparing regulatory submissions
- Balancing sponsor generated queries
- Taking responsibility for study cost efficiency
- Preparation and review of study documentation and feasibility studies for new proposals
- Potential to assist in training and mentoring fellow CRAs
- Centrally reviews clinical data at aggregate level, using analytic reporting tool(s) to support the identification of risks and data patterns/trends.
- Reviews clinical and external data for subjects enrolled in clinical research protocols based on functional plans.
- Mitigates risks by using signal detection and quality indicators.
- Communicates and triages issues to appropriate roles for follow-up and action to address root cause.
- Creates analytic outputs and presentations to facilitate data reviews and to support data-driven decision-making.
- Supports activities related to development of the Clinical Data Management Systems (CDMS).
- Provides input to applications, databases and systems used to monitor study data.
- Contributes to the development and maintenance of study documents specifying central monitoring strategy, approach and procedures on assigned protocols/projects.
- Leads or supports the clinical data review activities associated with a clinical trial and the delivery of data fit for analysis
- Collaborate with CTOM/COM/Regional COMs and on-site monitors to ensure they are well equipped with the details of site related risk/issue(s) to plan timely site intervention (site visits or telephonic contacts with sites) and to ensure more efficient and focused activities during monitoring visits.
- Supports and executes the data review activities, including data validation and analytics, that contribute to delivery of clinical data that meets agreed upon data integrity standards.
- Supports activities related to development of the clinical data management systems (CDMS), as needed.
- Supports and executes the data review activities, including data validation and analytics, associated with a clinical trial to ensure the end product of clinical data meets agreed upon data integrity standards.
- Detects risks and signals in the data using quality indicators.
- Performs root cause analysis of detected data issues.
- Reviews clinical and third party data for subjects enrolled in clinical research protocols based on edit specifications/ Integrated data review plan to facilitate data review.
- Maintains clinical data management related study documentation as appropriate.
- Manages third party data reconciliation and data review process.
- Ensures study and task metrics are tracked and communicated to the clinical data scientist, project team and functional management.
- Responsible for review of standard quality indicator review including root cause analysis for identification of underlying.
- Reviewing documentation and requirements for new projects and anticipating impact to Data Management (DM) standards/processes
- Collaborating with appropriate project teams as needed to stay abreast of and changes that could affect data collection, cleaning and/or transfers
- Understanding the requirements for study implementation and data collection
- Defining, running, and reviewing edit checks and resolving discrepant data
- Maintaining organized, complete, and up-to-date study documentation
- Creating or reviewing Transmittal Forms for a study, ensuring consistency with existing standards
- Keeping supervisor informed of project status
- Reviewing data and identifying errors/inconsistencies
- Developing study data specifications, including data transfer specifications, system configuration specifications, and data validations.
- Collaborating with appropriate project team to resolve data issues
- Tracking outstanding issues and following-up until resolution
- Preparing and validating ad hoc data listings as necessary
- Ensuring that system bugs and needs for enhancements are reported to the applicable Product Manager and that any stop-gap programming is approved
- Perform Regulatory, Start-up and Maintenance activities according to applicable regulations, SOPs and work instructions. Distribute completed documents to sites and internal project team members.
- Prepare site regulatory documents, reviewing for completeness and accuracy.
- Review, prepare and negotiate site contracts and budgets with sites, if applicable.
- Ensure accurate completion and maintenance of internal systems, databases and tracking tools with project specific information
- Review and provide feedback to management on site performance metrics
- Review, establish and agree on project planning and project timelines. Ensure monitoring measures are in place and implement contingency plan as needed.
- Inform team members of completion of regulatory and contractual documents for individual sites.
- Review, track and follow up the progress, the approval and execution of documents, including contracts, regulatory, ethics, ICF and IP Release documents, in line with project timelines.
- Provide local expertise to RSU leads and Project team during initial and on-going project timelines planning.
- Perform quality control of documents provided by sites.
- May have direct contact with sponsors on specific initiatives.
- May perform Site Selection Visits if a trained monitor.
- May participate in feasibility and/or site identification activities
- Prepare budgets for proposals by interacting with operational departments to coordinate budgets and assumptions.
- Work with operational, legal, financial, and business development (sales) staff to maintain and improve proposals documents.
- Consistently, meeting deadlines and able to assist others.
- Coordinate and process information delivery for the budget and proposal.
- Chair kick-off and resource meetings and coordinate the participation of appropriate personnel in the proposal development process
- Responsible for compiling, editing, and owning the entire process from request to delivery, associated with the proposal.
- Assist with continuous departmental process improvements and participate in special projects periodically assigned, in addition to day-to-day duties
- Coordinate and participate in negotiations and discussions with clients as required
- Recognize, exemplify, and adhere to Sponsor/CRO values which centre around commitment to People, Clients, and Performance.
- Authors clinical regulatory documents following defined templates.
- Independently builds and expands capabilities to work on increasingly complex assignments.
- Understands the importance of consistency and quality level for all documents.
- Establishes network of communication and continuously builds these relationships with stakeholders to facilitate efficient execution of assignment.
- Participates in document planning and review meetings.
- Proactively raises and discusses concerns/ issues in an open and timely manner within the global Medical Writing Team
- Promotes high scientific and medical writing standards by pointing out obvious flaws and proposing alternatives.
- Interprets and communicates clinical data clearly and succinctly and at an appropriate level for the audience
- Responsible for scientific medical writing and producing high quality documents
- Preparation of Protocols, Informed Consent Forms, Case Report Forms, and other protocol related documents as per applicable regulatory requirements
- Responsible for updating the protocol
- To define & drive the CSV strategy / plan to achieve the validated status during project stage as per the SOP
- Perform Risk assessment and carry out Risk based testing of Computerized System
- Preparation and Review of Validation deliverables such as URS, GxP, VP, Specifications documents(SDS, FS, SCS), IQ Protocols and Scripts, OQ Protocols and Scripts, PQ Protocols and Scripts, TM and VSR as per the SOP
- Execution/Support in execution of IQ, OQ and PQ test scripts
- Co-ordinate with Vendor and System owner to carry out Validation activity of Computerized Systems and Software
- Responsible for achieving & maintaining the validated status of in scope computerized systems
- Other Responsibilities assigned by Reporting Manager or Designee
- Periodic review of all GxP Software in Company
- Responsible to response to Audit query for the software by Regulators and Sponsors
- Responsible to face Internal Audit for Software
- Active member for External Audits faced by Company
- Assists with providing logical support for the operations of clinical research protocol automation for all phases of trial
- Streamlines process of protocol digitization
- Substantiates and provides reposes for various queries required in automation
- Maintains the schedule and track frequently used techniques and help in building a strong team of SME group
- Provides quality assurance and quality oversight
- Works closely with Data Scientists and Doctors by establishing and maintaining the transparency on various query requirements
- Managing complete sales by developing new business opportunity, commercial negotiation, and contract closure and revenue collection.
- Reviewing the technical agreement, MSA, NDA/CDA, commercial agreement quote/Rate contract.
- Resolving commercial query for internal external customer, through regular interaction.
- Prepare budget, business sales projection, strategic planning, and executing new business strategy in the market.
- Promote the company's products/services addressing or predicting clients objectives.
- Developing and sustaining solid relationships with company stakeholders and customers
- Assist with establishing and maintaining a strong partnership with Operational management assigned partners, other Quality and business functions (e.g., Risk Management, Project Quality & Risk Leads) and with Line Manager to ensure continuous improvement and regulatory compliance
- Perform other Quality related tasks as assigned
- Assist in supporting audit/inspection preparation including helping team set priorities and reviewing key documents
- Provide onsite (office) or remote support as needed
- Lead project team to investigate root causes
- Facilitate development of robust CAPA
- Respond to consultancy requests to enable project teams to deliver firsttime quality
- Review and propose SOP deviations using ICH-GCP, Sponsor processes, regulatory requirements
- Consult SMEs including other quality experts when necessary to resolve issues
- Review and investigate possible SOP deviations as requested
- Review audit/inspection responses prior to final QA review if necessary
- Contribute to critical issue resolution by providing expert quality advice and direction
- Sharing of Quality information internally
- Work with Quality and Operational management to identify risk areas
- Complete assigned Risk Evaluations including; support with identifying relevant questions & identifying project types to use during the assessment, discussing the results, defining suggested actions and providing input to a presentation of the results.
- Audit/inspection support: Represent Sponsor Quality and ensure the partnership /project team are prepared for audit, supported throughout and reviewing audit responses
- Prepare and update Annotation List, Data Validation Plan, Edit Check Specifications, and Database/ Data Entry Screens as per study requirements.
- Preparation of Operational Manual (EDC Trials)
- Prepare CRF Filling Guidelines.
- Perform query management activities per study requirements including the manual queries raised by data entry team and medical coder; i.e. query generation and resolution.
- Quality Check form updates and reverification.
- Filling the data in 2 CRF(s) or data base testing.
- Validate the external data after uploading into the database.
- SAE and Lab data reconciliation.
- Update Self Evident Correction queries in Self Evident Correction Form per study requirements.
- Update reports and trackers on regular basis or as per study requirements.
- Any other duties as assigned by supervisor or department
- Providing clinical research support to investigators to prepare for and execute assigned research studies, including: Review study protocols, Case Report Forms (CRFs), other study-specific documents, and electronic data capture systems used to record clinical research data
- Attend all relevant study meetings
- Collect and submit regulatory/ethics documentation as required by relevant regulatory bodies governing the conduct of clinical research
- Recruit and screen patients for clinical trials and maintain subject screening logs
- Orient research subjects to the study, including the purpose of the study, procedures, and protocol issues such as timelines for visits
- Design and maintain source documentation based on protocol requirements
- Schedule and execute study visits and perform study procedures
- Collect, record and maintain research subject study data according to study protocol and SOPs, preserving quality control for content, accuracy and completeness
- Handle lab testing and analysis, including preparation of specimen collection tubes and lab logistics
- Correspond with research subjects and troubleshoot study-related questions or issues
- Assist with study data quality checking and query resolution
- Record, report and interpret study findings appropriately to develop a study-specific database
- Assist investigator in verifying that research study objectives are met on time, within budget and according to applicable protocol requirements, clinical research regulations and quality standards
- Assist in maintaining adherence to investigator site staff training requirements by auditing and maintaining training records
- Prepare for and attend study monitoring visits, study audits, and regulatory inspections with clinical research regulatory agencies
- Maintain Essential documents and complete study related logs
- Assist Clinical Research Associates (CRAs) and Regulatory and Start-Up (RSU) team with accurately updating and maintaining clinical documents and systems (e.g., Trial Master File (TMF and CTMS)) that track site compliance and performance within project timelines.
- Assist the clinical team with the preparation, handling, distribution, filing, and archiving of clinical documentation and reports according to the scope of work and standard operating procedures.
- Assist with periodic review of study files for completeness.
- Assist CRAs and RSU with preparation, handling and distribution of Clinical Trial Supplies and maintenance of tracking information.
- Assist with the tracking and management of Case Report Forms (CRFs), queries and clinical data flow.
- Act as a central contact for the clinical team for designated project communications, correspondence and associated documentation.
- May accompany CRAs on site visits to assist with clinical monitoring duties upon completion of required training.
- Initiates feasibility intelligence collection based on study needs.
- Assists in vetting local sites, including research from internal and external sources.
- Gain an understanding of identifying risks to quality and compliance and develop and implement mitigation plans.
- Will recommend site lists to suit the needs of the feasibility and to support the site development strategy.
- Document all communication attempts and follow ups associated with the site and e-file essential documents.
- Communicates with the sites after survey review to clarify responses, as needed.
- Ensures compliance with the Feasibility schedule and raises concerns to Manager. Reviews status of sites with management weekly.
- Entry of feasibility and maintenance of key data into CTMS systems that supports predictability, randomizations, forecasting, and other key performance measures within assigned therapeutic areas.
- Collaborate with sponsor teams involved in the Study Start-Up process.
- Drug safety or Pharmacovigilance Associate
- Drug Safety and PV scientist or specialist
- Drug safety or PV manager
- Safety or PV reviewer
- Pharmacovigilance Quality Compliance
- Clinical Quality specialist
- Medical Safety scientist/specialist/reviewer
- QPPV or PvOI
- Clinical trial project safety associate (reviewer or specialist)
- MedDRA coder
- Pharmacovigilance Auditor or PV Inspection readiness officer
- Safety or Pharmacovigilance Physician (medical director, MD/MBBS, IMG)
- Safety Compliance Writer
- Case processing specialist
- Risk management manager
- Signal management specialist
- Periodic reporting specialist
- Regulatory affairs safety specialist
Cliniminds – www.cliniminds.com – Established in 2004, offers job-oriented, upskilling, and professional training programs in the life sciences sector. Our programs cover areas such as clinical trials, pharmacovigilance, data management, business analytics, medical writing, regulatory affairs, and other specialized fields. Cliniminds has an alumni of over 11,500+ professionals from India, the US, Europe, the Middle East, Africa, and Latin America. Our programs are accredited and certified by the Life Sciences Sector Skill Development Council, Ministry of Skill Development & Entrepreneurship, and the Accreditation Council for Clinical Research Education, USA.
Cliniminds has seasoned senior industry experienced faculty, an experienced placement team, robust IT platform, ERP/LMS, and business development teams. Cliniminds has a strong international footprint with active students from 20+ countries, including developed markets like the US and Europe. Cliniminds has recently signed an MoU with the leading Management & Science University, Malaysia, to offer Cliniminds training programs at their Malaysian campus.
Cliniminds provides corporate training solutions to leading corporates like Oracle, Birla Soft, Ultragenic, Bio Pharma, USA, and many more.
Cliniminds Academics Advisory Board
Cliniminds Academics Advisory Board consists of eminent professionals from the Life Sciences, Healthcare & Pharmaceutical industry. These members bring different areas of expertise in the life sciences sector.
Cliniminds work very closely with the Academic Advisory Board on industry-academia issues, program upgrades, aligning programs with industry requirements.
Some of the key members are:
- Dr. Suneet Sood, Professor of Surgery, International Medical University, Malaysia
- Mr. Paul Benninger, Alliance Director – Global Business Excellence, Alexion Pharmaceuticals Inc. Canada
- Mr. Amit Ananpara, Managing Director & Executive Member of the Board, Innoplexus Consulting, Germany
- Dr. Sumit Verma, Director, Soterius Life Sciences, USA
- Dr. Amit Garg, Senior Director, Medical | Clinical | Strategy & Planning | Training & Education, Terumo India
- Dr. Sandeep Bhatia, Vice President, Healthcare Ecosystem, Innoplexus
- Dr. Arun Gupta, Director, AyuSwasth, India
- Mr. Sanjay Bansal, Founder & Managing Partner, Aurum Equity Partners LLP, India
- Dr. Mohd Fadli Bin Mohd Asmani, Deah, School of Pharmacy, Management & Science University, Malaysia
- Dr. Devesh Kumar, Founder & Director, IR Innovate Research
- Dr. A. Indani, Medical Devices Expert
Cliniminds Delhi NCR - Cliniminds Delhi NCR - Noida B 602, KLJ Noida One, 6th Floor, Sector 62, Noida, Uttar Pradesh, India 201309
Cliniminds Mumbai - Awfis Vashi, 18th Floor, Cyber One, Sector 30A, Vashi, Mumbai, India, 400703
Cliniminds Bangalore - BHIVE Workspace Platinum Indiranagar, 271, 6th Main Rd, HAL 2nd Stage, Motappapalya, Indiranagar, Bengaluru, Karnataka 560038
Cliniminds Kerala 1 - Inspirit Safety Solutions (Head Office), 5th floor (Space V2), KSRTC Bus Terminal Complex, Thiruvalla, Pathanamthitta, Kerala, India, 689101
Cliniminds Kerala 2 - Inspirit Safety Solutions (Ernakulam Branch), V1 Plaza, 4th floor, Old Railway Station Rd, Kacheripady, Ernakulam, Kerala, India, 682018
Cliniminds West Bengal - Netaji Subhas Chandra Bose Cancer Hospital, 3081 Nayabad, Near 1B Bus Stand, New Garia, Kolkata, West Bengal, India, 700094